We focus on the biochemistry, toxicity, and detection of misfolded proteins in Alzheimer disease and other neurodegenerative diseases. We draw our scientific questions from unsolved problems patients with these diseases face. Current projects include the following:
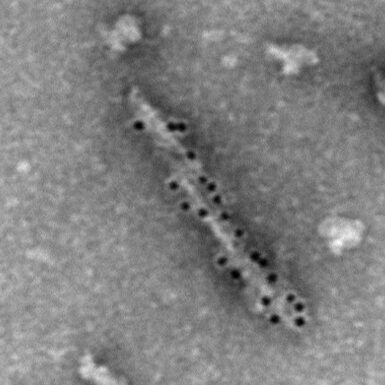
Biochemistry and structure of diffusible Aβ fibrils in human brain. Freely diffusing aggregates of Aβ peptide have been known since the 1990s to correlate better with dementia than amyloid plaques. We showed recently that the structures of these aggregates (sometimes called protofibrils or high molecular weight oligomers) are the same as those of aggregates in amyloid plaques. We next wish to define binding partners of the diffusible fibrils in human brain that might cause toxicity and model the kinetics of their diffusion in mice.

Causes of ARIA. Amyloid related imaging abnormality (ARIA) is the worst side effect of anti-amyloid monoclonal antibodies for the treatment of Alzheimer disease such as lecanemab and donanemab. ARIA is usually asymptomatic but is catastrophic in rare cases. The cause is ARIA is unknown but probably relates to immune activation around Aβ aggregates within blood vessel walls, also known as cerebral amyloid angiopathy (CAA). We are developing new techniques to measure antibody affinity to human brain Aβ from plaques and CAA to determine why some antibodies cause more ARIA, and to design ones that don’t.
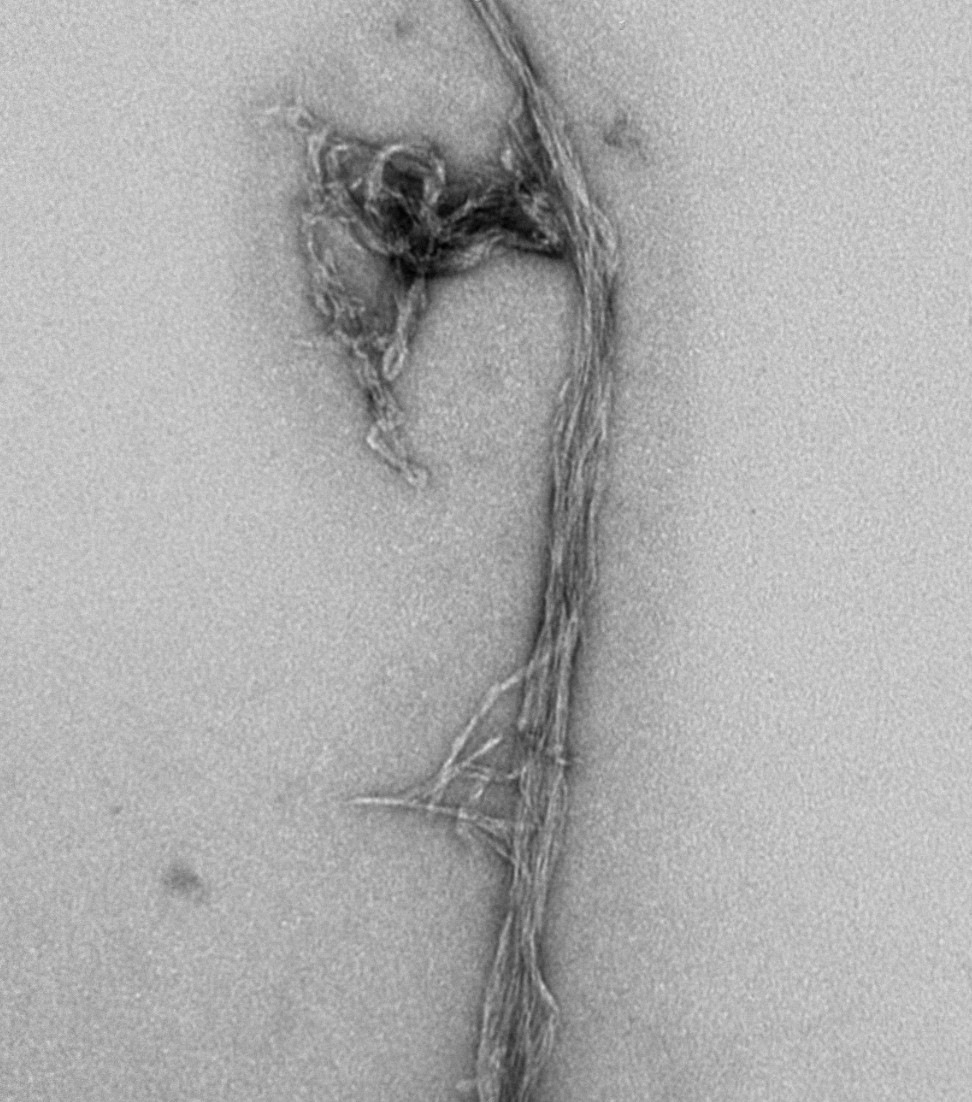
Biomarkers of non-AD neurodegeneration. The development of modern treatments for Alzheimer disease was enabled by accurate biomarkers. Knowing that Alzheimer disease co-occurs with other misfolded protein diseases as often as it occurs in isolation, and knowing that clinical diagnosis alone is inaccurate, biomarkers for non-Alzheimer disease pathologies are necessary to create accurate treatments for dementia. Supported by the Erica S. Johnston Frontotemporal Dementia Research Fund, we are aiming to create biomarkers of TDP-43 pathology such as FTLD-TDP and LATE.